Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hirade, Tetsuya
JPS Conference Proceedings (Internet), 25, p.011021_1 - 011021_2, 2019/03
Irradiation of water produce some reactive species such as OH radical. OH is formed by the reaction of a water cation and a water molecule just after ionization. On the other hand, a high energy positron injected in water will form cations and excess electrons even at the end part of the track. And hence, some positrons can form Positronium (Ps) with one of the excess electrons. The electrons in OH and Ps used to be in a same orbital in a water molecule before ionization of that water molecule. Therefore they were singlet at the time of the ionization. Every electron have each own hyperfine coupling constant after ionization. In water, reaction between Ps and OH, such as radical reaction or spin conversion, is possible. Therefore, quantum beats on these reaction can occur and the frequency of quantum beats will indicate the hyperfine coupling constant of OH which depends on the structure around OH. Therefore it is becoming possible to discuss the structure of water and reactivity of OH in the structure.
Suzuki, Satoru; Kawamura, Katsuyuki*
JNC TN8400 2001-005, 41 Pages, 2001/04
A correlation between molecular structure and a vibrational spectrum of interlayer water in Na-smectite was investigated by means of Molecular Dymamics (MDs) simulations. Detailed comparison of simulation results with IR spectroscopic observations for the water-smectite system indicated good agreement. Internal vibrational spectra of water were obtained by the Fourier transformation of velocty auto-correlation function of hydrogen atom. A stretching vibrational spectrum of interlayer water consisted of a broad band with a peak top around 3400cm and a sharp peak around 3650 to 3700cm
and a sharp peak around 3650 to 3700cm . The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O
. The fomer broad band was assigned to O-H vibrations between water molecules as bulk water, while the latter band was attributed to O-H ones oriented to siloxane surface through hydrogen bonding. The hydrogen bond distance, determined as the shortest O-O distance by the radial distribution function (RDF), revealed that hydrogen bond distance between water and siloxane surface (O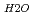 -O
-O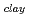
 3.0
3.0  -O
-O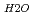 = ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
= ca. 2.8 AA ). These results suggested that interaction between water molecule and siloxane surface weaker than that between water molecules, although they were forced to be oriented.
Ozaki, Yusuke; Kohashi, Akio; Onoe, Hironori
no journal, ,
no abstracts in English
Hirade, Tetsuya
no journal, ,
A high energy positron injected in water will form cations and then OH radical will be formed. On the other hand, an excess electron formed by the inization and positrons can form Positronium (Ps). The electrons in OH and Ps used to be in a same orbital in a water molecule before ionization of that water molecule. Therefore they were singlet at the time of the ionization. Every electron have each own hyperfine coupling constant after ionization. In water, reaction between Ps and OH, such as radical reaction or spin conversion, is possible. Therefore, quantum beats on these reaction can occur and the frequency of quantum beats will indicate the hyperfine coupling constant of OH which depends on the structure around OH. At 15 C and 10
C and 10 C, quantum beats were observed and it indicated that water structure changed at these temperature range.
C, quantum beats were observed and it indicated that water structure changed at these temperature range.